The C-cl Bond Is Best Described as
Video Explanation Solve any question of Haloalkanes and Haloarenes with- Patterns of problems. The electrons are withdrawn by the chlorine atom.

Sefire Stele 8th Century Bc Described As The Best Extra Biblical Source For West Semitic Traditions Of Bible History Black Hebrew Israelites Paleo Hebrew
1095 The reason an ionic bond forms can best be described as.

. Draw the Lewis structure of CHCl₃ and then determine the ideal bonding angle s for the Cl-C-Cl bond s. 4 The water loses heat and the block gains heat until both are at the same temperature between 100C and 900C. 6 Given the reaction.
So In this article I tried to cover the nonpolar nature of Carbon Tetrachloride molecule CCl4. The lone pair on chlorine are dispersed throughout the benzene ring by resonance. D LiCl C KCI 6.
Chloroform CHCl₃ is an important laboratory solvent with a relatively high vapor pressure at room temperature. A characteristic of ionic solids is that they A. Which statement describes a multiple covalent bond.
The C-F bond is shorter and stronger than the C-Cl bond and shorter than single CN and CO bonds. However the C-Cl bond is a polar covalent bond but the four bonds cancel the polarity of each other and form a nonpolar CCl4 molecule. H 2 Cl 2 2HCl Which of the following statements best describes in this reaction.
1 breaking HH bonds releases energy 2 forming HCl bonds absorbs energy. The C-Cl bond in the butyl chloride CH 3-CH 2-CH 2-CH 2-Cl is polarized due to electronegativity difference. The Cl-Cl bond of Chlorine is best described how.
The CCl bond in C 6 H 5 Cl is shorter than in CH 3 Cl and therefore stronger. Which formula represents a mo ecu ar compound. The electron configuration of a carbon atom has how many electrons unpaired.
Option C is correct. The bonds in BaO are best described as A. The Cl Cl bond is best described as A nonpolar covalent B polar covalent C ionic from CHM 241 at Northern Virginia Community College.
B the transfer of electrons from one atom to another. Question-1 The inductive effect can be best described as. Between 100C and 900C.
Four electrons are shared. H2 Cl 2 2HCl Which statement best describes the energy change as bonds are formed and broken in this reaction. In this study we measure the photofragment angular distributions for each C-Cl bond fission channel and the spin-orbit state of the Cl atoms produced.
Have low boiling points. A Two electrons are shared. C the attraction that holds the atoms together in a polyatomic ion.
The CCl bond is best described as A Nonpolar covalent B Polar covalent C Ionic from CHEM 1240 at Wayne State University. It is best described as _____. If you guys have any questions regarding this you can ask that in the comment section below.
Based on these values and on consideration of molecular geometry the Si-Cl bond is _____ and the molecule is. P and Cl D. H and Cl 8.
A The forming of the H-Cl bond releases energy b The forming of the H-Cl bond absorbs energy c The breaking of the H-H bond releases energy d The breaking of the Cl-Cl bond releases energy. The approximate bond angles for the C-C-H bonds in the following molecules are. Pages 32 This preview shows page 4 - 14 out of 32 pages.
Orbitals that are equivalent in energy are referred to as. How many distinct p orbitals exist in the 2nd electron shell. Contains only ionic bonds solid A contains only ionic bonds and solid B contains only covalent bonds 5.
A chemical bond formed when two atoms share one pair of electrons is a _____ bond. C Two electrons are transferred. End question 2 the clcl bond of chlorine is best.
An ionic bond is best described as A the sharing of electrons. The strength of the C-F bond is due to its partial ionic character and due to electrostatic attractions between the partial charges on. Course Title CHEM 2201.
B the ability of atom or group to cause bond. Have high melting points B. D Four electrons are transferred.
The electronegativity values for Si and Cl are 18 and 30 respectively. This gives the CCl bond a double bond character. For the methyl cation CH3 the geometry H-C-H bond angle and hybridization of the carbon atom is best described as.
End QUESTION 2 The ClCl bond of chlorine is best described how A Nonpolar. A the conjugation of σ-bonding orbital with the adjacent π-orbital. School University of New Haven.
What is the expected bond angle for the C-C-O bonds in the molecule below. The data shows bond fission leading to the production of ground-state. 50 The C Cl bond is best described as A Nonpolar covalent B Polar covalent C from CHEM 200L at University of Santo Tomas.
D the attraction between two nonmetal atoms. 33 Given the reaction. Trigonal planar 120 degsp2.
That work evidenced two competing C-Cl bond fission channels tentatively assigning them as producing ground- and excited-state methoxy carbonyl radicals. The C-Cl bond is best described as A. Covalent because valence electrons are shared B.

Fabulous Vintage Sherlock Movie Posters Classic Films Posters Poster Vintage Retro Sherlock Poster

Alarmed Canvas Print Canvas Art By Denny Bond Stretch Canvas Print Bond

Dual Core With Analog Amp Digital Display Water Resistant 1pcs X Wrist Watch Case Material X3a Stainle Luxury Watches For Men Watch Design Casio Protrek

Covalent Bonds Flashcards Quizlet

Chapter 5 Tb Flashcards Quizlet

Organic Chemistry 331 Sapling Learning Ch 3 Flashcards Quizlet

Chapter 5 Tb Flashcards Quizlet

Chapter 5 Tb Flashcards Quizlet

Honors Chemistry Unit 6 Test Flashcards Quizlet

Chapter 5 Tb Flashcards Quizlet

Covalent Bond Definition Properties Examples Facts Britannica

Covalent Bonds Flashcards Quizlet

Chapter 2 Basic Aspects Of Biochemistry Organic Chemistry Acid Base Chemistry Amino Acids Protein Structure And Function And Enzyme Kinetics Flashcards Quizlet

Chem Lewis Structure Flashcards Quizlet

Aa X Pkmn Epic Battle Apollo Justice Phoenix Wright Best Crossover
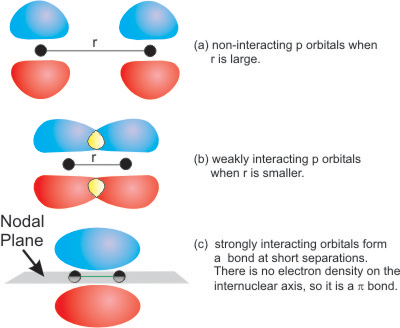
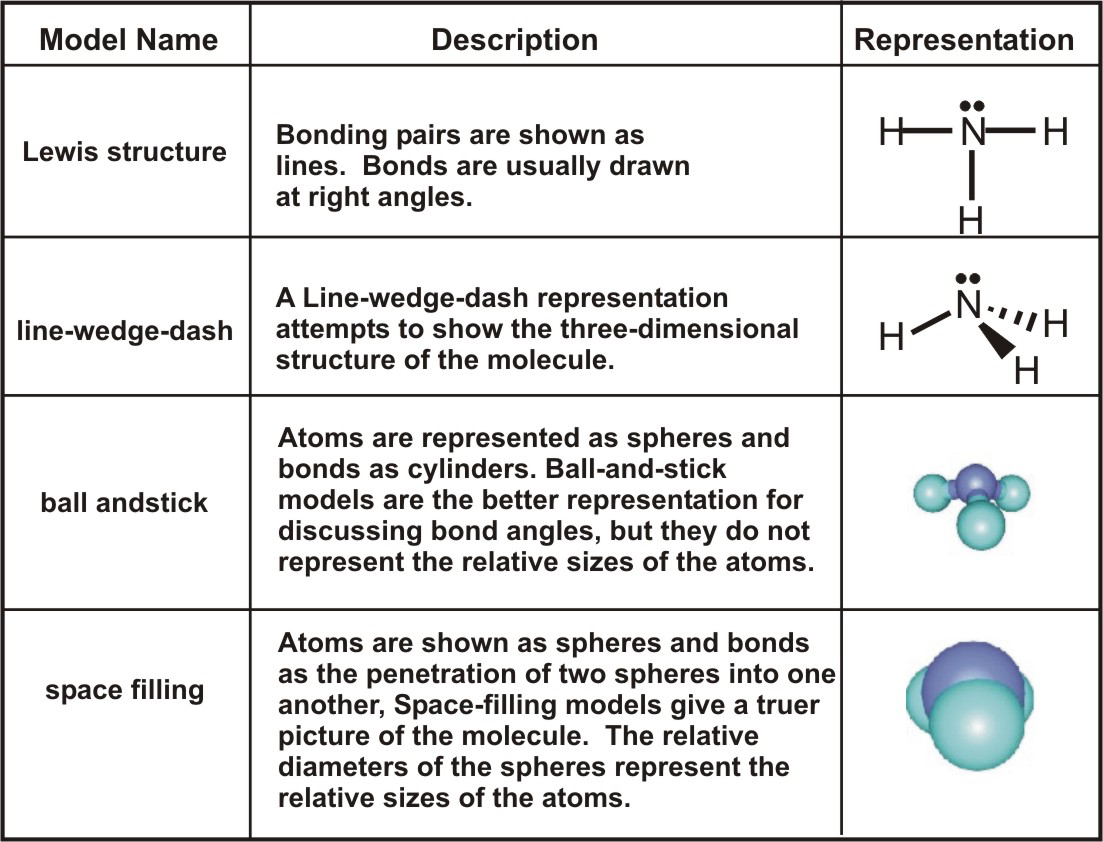
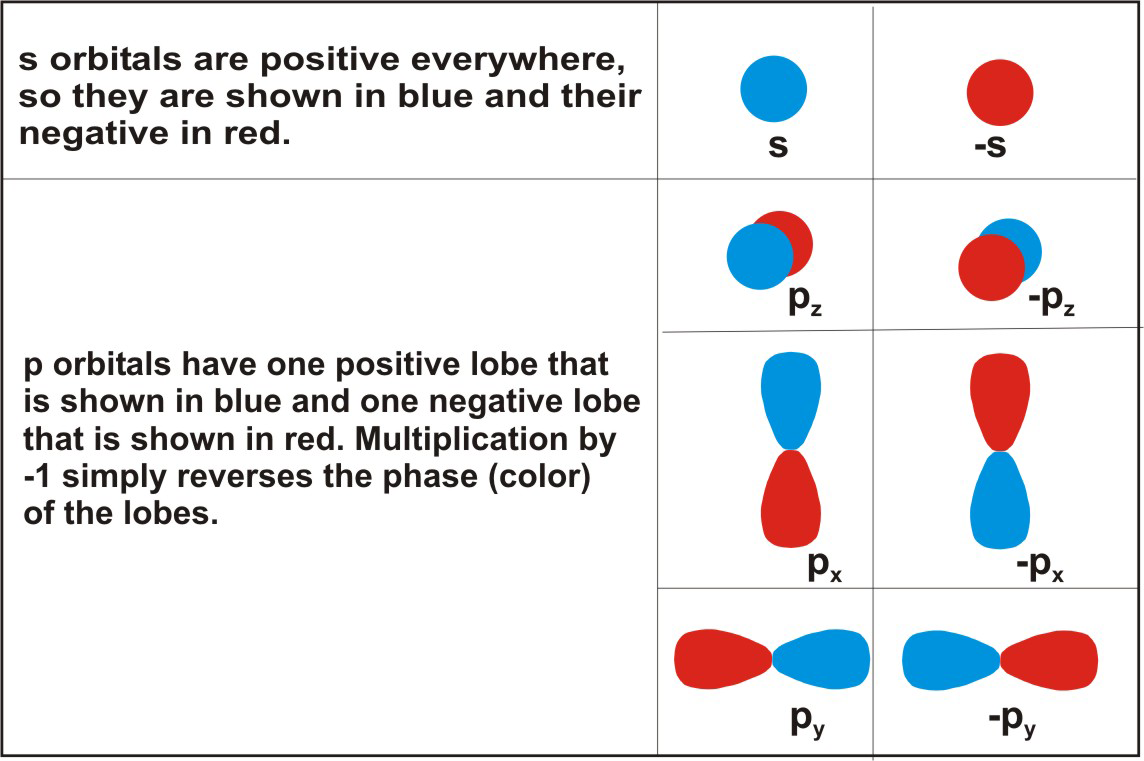
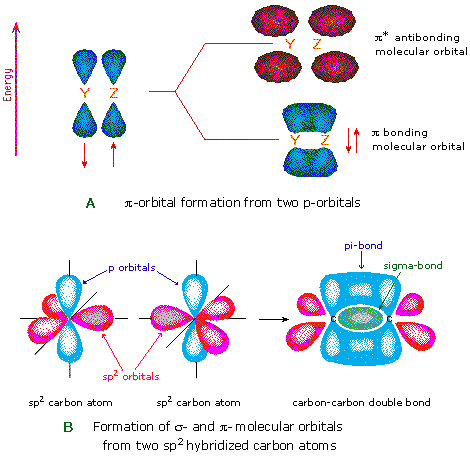
Comments
Post a Comment